Pharmacovigilance service in Bangladesh
Pharma DRA’s technical team submits adverse drug reaction monitoring (ADRM) form in DGDA on behalf of the foreign manufacturers. Monthly ADRM report submission to DGDA is mandatory for the pharmaceuticals (drug) manufacturers and finished medicine importers.
We provide the PV services in Bangladesh:
- SOP on Pharmacovigilance.
- Periodic Aggregate Reports.
- Adverse Events Case Management.
- Computerized System (s) for PV activities.
- Digitally Archive and Storage of documents.
- Signal Detection and Emerging Safety Issues.
- Interaction between PV and other Departments.
- Corrective Actions and Preventive Actions (CAPA).
- Regulatory Intelligence in PV and Disaster Recovery.
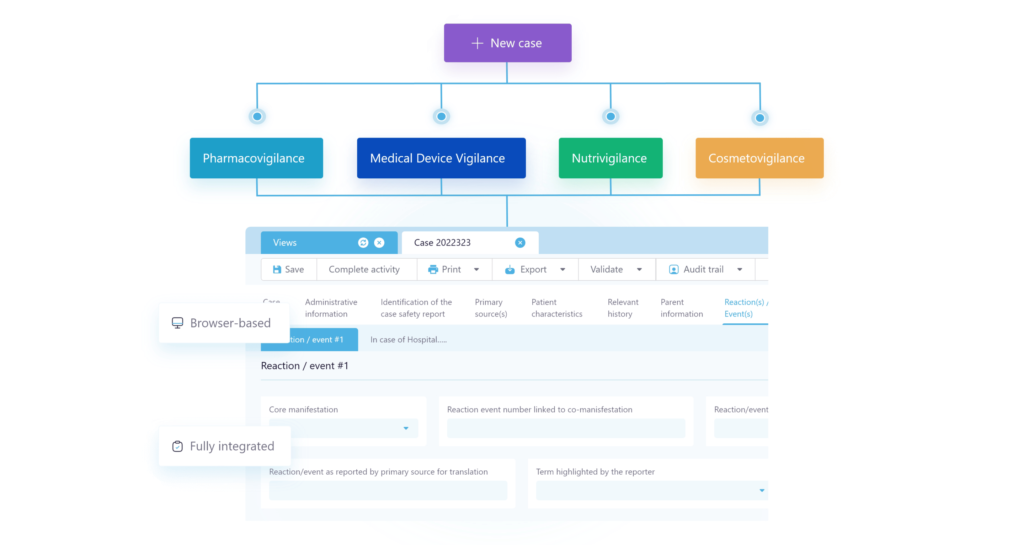
Cloud safety database software for pharmacovigilance compliance, medical device vigilance, cosmetovigilance and nutrivigilance.
- Simplify & Speed up case intake
- Gain case creation efficiency
- Enhance case analytics
- Improve signal detection
- Seamless system connectivity
- Automate ICSR Processing
- Gain accuracy and authencity